CellSync Creams Market Sees Strong Growth Across APAC, Europe, USA, and Saudi Arabia Driven by Premium Skincare Adoption
MD, UNITED STATES, February 6, 2026 /EINPresswire.com/ --
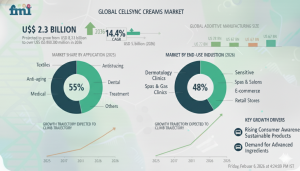
The global CellSync creams market is entering a high-growth, compliance-driven expansion phase, projected to grow from USD 2.3 billion in 2026 to USD 8.8 billion by 2036, according to Future Market Insights (FMI). This represents a robust 14.4% CAGR, driven by tightening claims governance, premiumisation of post-procedure skincare, and the formalisation of aftercare regimens across dermatology clinics, medical spas, and controlled digital channels.
Growth in the CellSync creams category is increasingly anchored in regulatory uplift and protocol-led consumption rather than discretionary beauty spend. Authorities across major markets are strengthening substantiation and post-market oversight requirements, reshaping how premium skin repair and renewal products are positioned, documented, and distributed. As a result, CellSync creams are evolving from optional adjuncts into structured components of professional skincare protocols.
Claims Governance and Compliance Drive Structural Premiumisation
In the United States, the Modernization of Cosmetics Regulation Act of 2022 (MoCRA) has introduced federal requirements including serious adverse event reporting, facility registration, and product listing. These changes are pushing responsible persons and manufacturers to formalise post-market surveillance, documentation, and label governance, aligning premium cosmetic skincare more closely with regulated health product operating models.
In China, the Standard for the Evaluation of Efficacy Claims of Cosmetics, effective May 2021, has raised the cost of substantiation and strengthened requirements for claim disclosure. This framework favours platforms that can fund clinical-style testing at launch, structurally advantaging CellSync brands built around measurable renewal endpoints rather than loosely framed regenerative narratives.
Physician-Dispensed Channels Reinforce Protocol-Based Demand
Physician-dispensed brands are using portfolio cadence and clinic-first strategies to defend and expand premium channels. In April 2024, Allergan Aesthetics, an AbbVie company, launched new SkinMedica acne products, reinforcing provider-led care and protocol-driven usage.
This clinic-based ecosystem links CellSync creams directly to in-office procedures and recovery pathways, converting aesthetic volume into structured, repeatable aftercare regimens. Repeat purchase is increasingly tied to office recommendation, patient education, and post-procedure recovery protocols, reinforcing CellSync creams as long-term regimen components rather than one-time retail purchases.
Cell Renewal Creams Lead Product Mix
Cell Renewal Creams account for approximately 47.5% of total market share in 2026, reflecting their compatibility with measurable cosmetic endpoints and daily-use regimen placement. Renewal creams align most closely with substantiated cosmetic claims and avoid regulatory escalation into drug, biologic, or quasi-drug pathways.
Regulatory regimes in China, the United States, and the European Union favour renewal formats built around well-documented cosmetic actives, enabling brands to compress time-to-market and scale within existing governance stacks. Commercially, renewal creams also occupy the daily recovery and maintenance slot following in-clinic procedures, making them the most easily bundled and replenished protocol component.
Online Direct-to-Consumer Leads Distribution
Online direct-to-consumer channels lead distribution, reflecting the growing importance of traceability, compliance, and education. MoCRA’s post-market obligations and similar governance frameworks internationally are encouraging brands to centralise sales, adverse event handling, label updates, and consumer communications through controlled digital ecosystems.
Direct channels reduce third-party relabelling risk, support faster dossier updates, and align with regimen-based replenishment models. As compliance and education requirements tighten, DTC platforms are becoming the preferred channel for premium CellSync cream distribution.
China and India Lead Global Growth
China represents the largest growth opportunity, with a projected 19.44% CAGR from 2026 to 2036, driven by efficacy-claim evaluation requirements and the ability of disciplined brands to fund testing and documentation at launch. The regulatory architecture rewards substantiated renewal positioning and supports rapid digital scale for compliant platforms.
India follows with an 18.0% CAGR, supported by the Cosmetics Rules, 2020 framework and maturing compliance infrastructure. As traceability, labelling, and import discipline improve, dermatology clinics and premium brands are scaling higher-priced recovery products and expanding direct digital fulfilment beyond tier-one cities.
Competitive Landscape: Protocol Brands Dominate
Competition is concentrated among physician-dispensed and premium protocol brands capable of defending cosmetic positioning while maintaining clinical credibility. FMI estimates SkinMedica as the largest global player in 2026, supported by AbbVie’s Allergan Aesthetics platform and clinic-first portfolio cadence.
North America remains the operational centre of the category, with SkinMedica, Alastin, and ZO Skin Health competing through dermatologist offices and medical spas, then extending retention through controlled online replenishment. Europe and Asia exhibit more fragmented competitive dynamics shaped by regional regulatory frameworks and claim substantiation regimes.
Subscribe for Year-Round Insights → Stay ahead with quarterly and annual data updates: https://www.futuremarketinsights.com/reports/brochure/rep-gb-31843
Request for Sample Report | Customize Report |purchase Full Report -https://www.futuremarketinsights.com/reports/sample/rep-gb-31843
Strategic Outlook Through 2036
The CellSync creams market is transitioning into a structurally embedded, compliance-governed premium skincare category. Growth will be driven by tightening claims oversight, expansion of protocol-based aftercare, and the increasing conversion of aesthetic procedure volume into recurring home-use regimens.
As regulatory scrutiny increases and the boundary between cosmetics and regenerative medicine tightens, market leadership will increasingly be defined by substantiated renewal claims, disciplined portfolio governance, and controlled distribution models. Suppliers that combine compliance readiness, protocol integration, and digital replenishment will be best positioned to capture sustained growth as the market advances toward USD 8.8 billion by 2036.
Explore More Related Studies Published by FMI Research:
Battery Powered Surgical Drill Market-https://www.futuremarketinsights.com/reports/battery-powered-surgical-drill-market
Cytotoxic Chemotherapy Market-https://www.futuremarketinsights.com/reports/cytotoxic-chemotherapy-market
Targeted Oncology Biologics Market-https://www.futuremarketinsights.com/reports/targeted-oncology-biologics-market
Chaperone-based Therapeutics Market-https://www.futuremarketinsights.com/reports/chaperone-based-therapeutics-market
Substrate Reduction Therapies Market-https://www.futuremarketinsights.com/reports/substrate-reduction-therapies-market
About Future Market Insights (FMI)
Future Market Insights, Inc. (FMI) is an ESOMAR-certified, ISO 9001:2015 market research and consulting organization, trusted by Fortune 500 clients and global enterprises. With operations in the U.S., UK, India, and Dubai, FMI provides data-backed insights and strategic intelligence across 30+ industries and 1200 markets worldwide.
Why FMI: Decisions that Change Outcomes- https://www.futuremarketinsights.com/why-fmi
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Have a specific Requirements and Need Assistant on Report Pricing or Limited Budget please contact us - sales@futuremarketinsights.com
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.